The Nordic Mentor Network of Entrepreneurship (NOME) is the first pan-Nordic mentor network for lifescience start-ups. Why is it a good idea for start-ups working in cancer?
Bjørn Klem has an answer. He is the General Manager of Oslo Cancer Cluster Incubator and point of contact for start-ups within the cancer field in Norway.
“Start-ups working in cancer need to access commercialisation expertise and investor networks. When looking for this, it is an advantage to seek in other Nordic countries where investors are experienced with cancer and biotech in general. Participating in NOME will also take you into their global network.” Bjørn Klem
Connecting with a mentor team
NOME is based on the mentoring principals of MIT’s Venture Mentoring Service. The fundamental principle is to connect first time entrepreneurs with a team of three to four experienced and skilled mentors to help them reach their goals and technology milestones.
From Boston to the Nordics, this is the first mentor network within life sciences that spans across all the Nordic countries.
In Norway, Oslo Cancer Cluster Incubator og the health incubator Aleap are coordinating start-ups with suitable mentors.
“Team mentorship, where mentees have a group of mentors, rather than single one-on-one mentorship, encourages more diverse thinking, cross-disciplinary approaches to ideas and problem solving, and it allows the access to professionals from different fields.” NOME Magazine Issue 1 2018
Norwegian mentors and start-ups
One of the Norwegian NOME mentors is Kari Grønås. She has extensive experience in drug development and commercialisation within the pharmaceutical industry.
You can listen to her (in Norwegian) in this video that was made by Oslo Cancer Cluster Incubator as the programme was just starting in Norway in 2017.
One of the Oslo Cancer Cluster members that have taken advantage of the NOME opportunity and mentors, is Nacamed.
Nacamed is a Norwegian spin-off company of Dynatec AS. The Nacamed technology is based on 10 years of research on silicon done by Dynatec engineering. According to the company webpage, this enables a production that can tailor particles with the desired physical attributes. With this, Nacamed aims to create a new generation of treatment methods.
Best in class-network
This video, made by Accelerate, explains the concept of NOME and the value it adds to the Nordic startup ecosystem.
The mentors are volunteering to share their knowledge and experience with new entrepreneurs within fields such as digital health, immuno-oncology and AI in healthcare. NOME mentors can give unbiased advice, provide strategic guidance, open their network and possible collaboration partners, as well as assisting in reaching key milestones.
The start-ups have to be best in class too. The local NOME partners evaluate the companies on the novelty of the science or technology, their high commercial potential as well as the strength and commitment of the founding team. Furthermore, strong IP or alternative protection strategies, market differentiation, and the impact NOME potentially can have on the company’s development are also taken into consideration.
Participation is free of charge and funded by the Novo Nordisk Foundation.
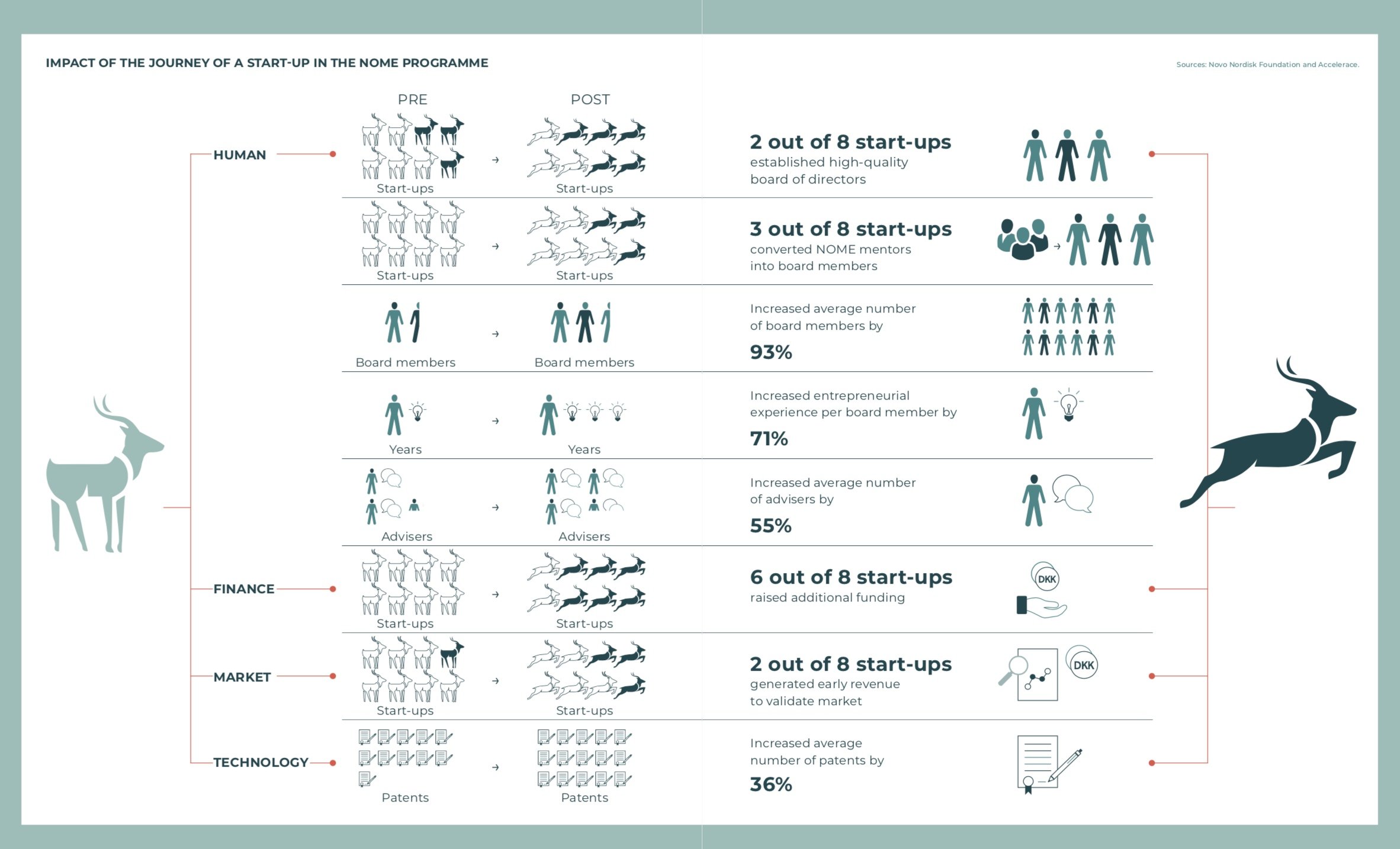
Source: The NOME Magazine, Issue 01, 2018
20 start-ups since 2016
Since 2016, 20 start-ups have joined NOME and of these two have graduated from the program. Graduation usually means the start-up has successfully raised funds for the coming few years and has engaged a formal board and therefore has less need for the NOME mentors.
The mentors either move on to work with other emerging companies or have been so excited about the potential of the company they have been working with that they have taken a seat on the board.
By the end of 2018, NOME had 50 mentors and 18 enrolled start-ups.
Mentors in immuno-oncology
In the NOME Magazine first edition, released in October, Carl Borrebaeck, professor at Department of Immuno-technology at Lund University in Sweden, is interviewed about his field of expertise, immuno-oncology and creating companies from his research. Borrebaeck is a founding mentor in NOME and has been part of the network for the past two years.
“People tend to think, that innovation just happens and that it will reach patients without any commercial drive. That is simply untrue.” Prof. Carl Borrebaeck
He continues to explain what is really needed to make health innovations happen:
“A combination of companies and academia is needed. Big pharma is always looking for the newest discoveries and ways they can collaborate in order to stay at the forefront of innovative research. The Nordics are highly innovative and they have a strong reputation globally. However, there are too few big pharma companies commercializing the science at the very early stages. This is often a major challenge for emerging companies who then have to seek funding not only in the Nordics but across Europe and the US to cover this funding gap.”
Mentors in artificial intelligence
NOME has mentors in several interesting life science fields. Lars Staal Wegner, the CEO of Evaxion Biotech, is another mentor. He started a company dedicated to using artificial intelligence, supercomputers, and big data to fight cancer and infectious diseases. In the NOME Magazine Wegner says:
“It is no longer the pharma industry or the companies producing the off-the-shelf drugs. It is the ones who own the data and know how to convert it to effect, the cloud-based giants that are half life science half tech. This is maybe 30-40 years into the future, but it is important already now to know that the tech evolution is not linear. It is exponential. We have reached an inflection point in tech. The industry doesn’t have five or ten years to toe the line. It is exploding.”
Artificial intelligence and machine learning are expected to have an unprecedented impact on how drugs are developed, their cost, and time to market, according to Wegner.
Nordic partnership
NOME is operated by Accelerace and funded by the Novo Nordisk Foundation. The initiative is represented in the Nordic region through partnerships in Sweden, Norway and Finland. In Norway, Oslo Cancer Cluster Incubator og the health incubator Aleap are coordinating start-ups with suitable mentors.
In the US, the California Life Sciences Institute (CLSI) is a new partner for NOME. In fact it is too new to have entered the overview below. CLSI is a non-profit organization which supports entrepreneurship, STEM education and workforce development for the life science industry in California. It is located in the San Francisco Bay Area.

Source: The NOME Magazine, Issue 01, 2018