Advancing Cancer Research in Norway: Eli Lilly’s SUNRAY-01 Study
Eli Lilly selected Norway as the site for its groundbreaking study project, SUNRAY-01, despite the recent year’s decline in applications for clinical cancer trials.
This study examines the efficacy of the drug candidate LY3537982 on advanced non-small cell lung cancer (NSCLC) with a specific genetic alteration. and represents a significant advancement in health research and treatment for patients with KRAS G12C mutations, potentially reshaping the treatment landscape for this specific patient group.
The KRAS G12C mutation is a specific alteration in the KRAS gene, often associated with certain cancers such as non-small cell lung cancer. This mutation plays a significant role in promoting the growth and spread of cancer cells. Researchers are exploring methods to block or inhibit the effects of this mutation.
Decline in trails
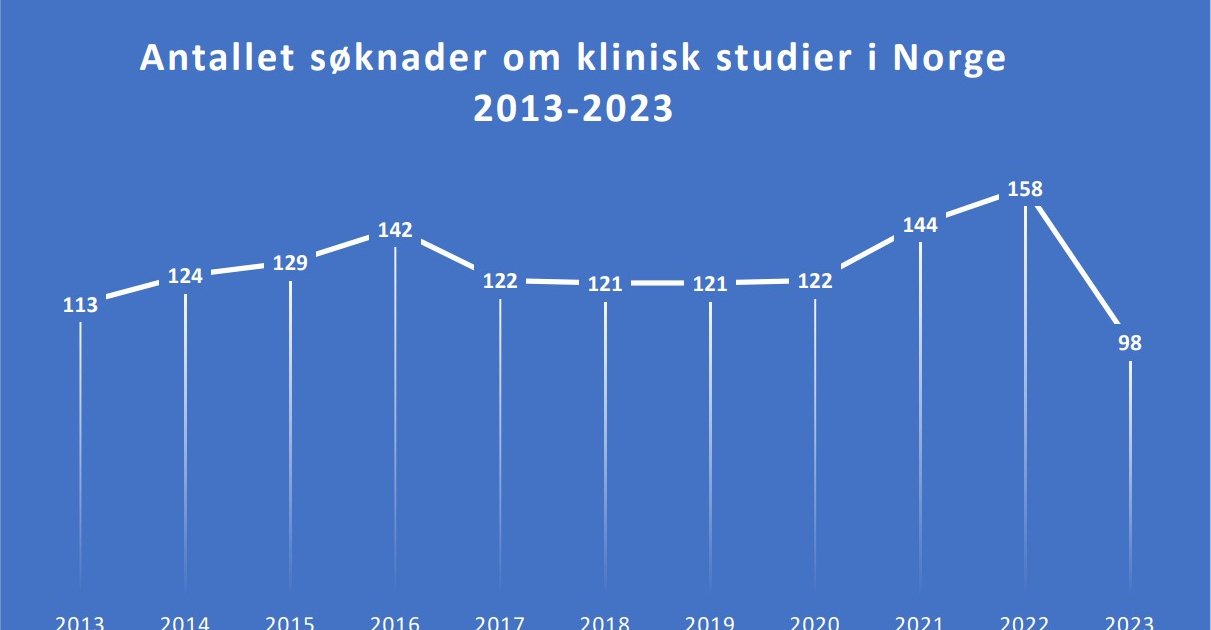
Recently, Norway has experienced a notable decline in the number of applications for cancer clinical trials, dropping from 158 in 2022 to 98 in 2023. Lars Petter Strand, Senior Medical Director for the Nordics at Eli Lilly, voiced concern, noting,

Lars Petter Strand. Photo Eli Lilly
“We observe that the number of cancer clinical trials in Norway has significantly decreased.” He highlighted the global trend of increasing clinical trials in countries like the USA and China, contrasting with the reductions in most European countries.
Norway’s participation in Eli Lilly’s SUNRAY-01 study indicates a positive shift. Lars Petter Strand attributed the decision to several favourable trends in Norway’s healthcare system, including initiatives like CONNECT, IMPRESS, InPRED, and NorTrials, which have enhanced infrastructure and processes, making Norway an appealing destination for clinical trials.
Positive outlook for patients
Bjørn Henning Grønberg, Head of Department for Translational Cancer Research at St. Olav Hospital, one of the 7 hospitals selected for this study, emphasized the importance of such studies, stating, “It is always welcome to offer study participation to our patients.” The proportion of lung cancer patients with KRAS mutations eligible for targeted treatment through this study exceeds those eligible for other targeted treatments.

One of the most exciting and significant aspects of this study is its focus on finding targeted treatments for KRAS mutations, which currently aren’t as effective as other options available.
Patients with this mutation respond to immunotherapy, unlike those with EGFR and ALK positives, making it an interesting combination to explore. However, in the past, this has been challenging, as the combination of KRAS inhibitors with immunotherapy was too toxic, says Grønberg.
Challenges and opportunities
Despite these positive developments, Norway encounters challenges in maintaining its attractiveness for clinical trials. Strand emphasized the importance of addressing barriers such as delayed introduction of new treatments, lengthy approval processes, and capacity constraints in diagnostic tools at hospitals.
The roadmap for the health sector, a strategic document guiding sector development, underscores the significance of clinical trials in health research. While the government has set ambitious goals for increasing clinical trials, collaboration across sectors and collective efforts are essential to address challenges hindering this vital part of medical research.
A roadmap for the health industry
Oslo Cancer Cluster General Manager Ketil Widerberg emphasizes that this new study aligns well with the Norwegian government’s aspirations for a national health industry and ongoing efforts at Oslo Cancer Cluster to foster innovation and collaboration within the cancer research field. It represents a crucial step towards advancing cancer care and supporting Norway’s health industry growth.
Widerberg stresses the importance of patients accessing the latest treatment, doctors and researchers gaining insights into the latest technology, and the development of the Norwegian health industry, as Norwegian centres of expertise gain international visibility.
Crucial collaborations
To attract more clinical trials to Norway, stakeholders must collaborate effectively, as Lars Petter Strand highlights. It requires creating sufficient resources in hospitals, facilitating efficient communication between the pharmaceutical industry and healthcare institutions, and streamlining startup processes. Improved communication between the pharmaceutical industry and hospitals is essential, as demonstrated by Eli Lilly’s collaboration with NorTrials during site recruitment for this study.
Collaboration between industry players, research institutions, and government bodies is crucial for advancing cancer research. Initiatives like NorTrials facilitate this collaboration, ensuring nationwide access to cutting-edge treatments, says Strand
The post Advancing Cancer Research in Norway: Eli Lilly’s SUNRAY-01 Study first appeared on Oslo Cancer Cluster.








