Under frokostmøtet Fremtidens kreftpolitikk kunne politikere fra fire av partiene på Stortinget diskutere om helsepolitikken deres er relevant for norske kreftpasienter.
Summary: During the breakfast seminar The Future of Cancer Politics four politicians discussed possible health- and cancer policies in their coming party programmes. The seminar was held in Norwegian.
Morgenen 9. april 2024 på Litteraturhuset i Oslo: Fire politikere fra går opp på scenen. De er Inger Noer (Venstre), Erlend Svardal Bøe (Høyre), Julianne Ofstad (FrP) og Marthe Scharning Lund (Arbeiderpartiet). I løpet av den neste timen skal de diskutere helsepolitikken sin for neste programperiode, men først tar de til orde for bedre samarbeid i egne rekker.
– Vi vet at flere vil få kreft og overleve kreft framover, og mye av kreftomsorgen er det kommunene som har ansvar for, så jeg er opptatt av at vi får et godt samspill mellom det som skjer nasjonalt, lokalt og regionalt, også politisk, sier Erlend Svardal Bøe, som er medlem i Helse- og omsorgskomiteen på Stortinget for Høyre.
Julianne Ofstad har gått fra å jobbe med helsepolitikk i stortingsgruppa til FrP til å bli lokalpolitiker i Oslo (hun er varaordfører), og hun oppfordrer også politikerne til å jobbe bedre på tvers av lokale og nasjonale politiske fora.
– Vi må være flinkere til å jobbe på tvers av lokalpolitikerne og de nasjonale og ha en mer helhetlig tilnærming til helsefeletet enn bare å se på hva skjer i kommunene og hva skjer i spesialisthelsetjenesten, sier hun.
Forebygging
Under seminaret viste vi fire korte videoer om relevante temaer for kreftpolitkken. I den første videoen snakker Sara Mjelva, Seksjonsleder for forebygging i Kreftforeningen, om nettopp forebygging.
I videoen stiller Mjelva spørsmålet: Hvordan vil ditt parti bidra til å forebygge sykdom i tiden som kommer?
– Forebygging handler om så himla mye mer enn røykeslutt. Jeg har vært helsebyråd i Oslo, og det handler om å tenke bredere i forebyggingspolitikken, om gode nabolag, å gi folk kunnskap om egen helse, å få med innvandrerbefolkningen og skape nettverk. Folkehelse er kjempeviktig, vi kommer til å knekke om vi ikke tar større grep rundt dette, sier Marthe Scharning Lund, som er leder for bystyregruppa til Arbeiderpartiet i Oslo, og sitter i programkomiteen i Arbeiderpartiet.
– Det dreier seg ikke bare om å få folk til å trene og spise sunnere. Folkehelsebombene er fedme, og alkoholforbruket har økt med 60 prosent på 30 år, og rødt kjøtt har en stor betydning for et bredt spekter av kreftsykdommer. Vi må regulerere når det gjelder usunn mat, og vi må forebygge der vi ser at det faktisk virker, og da trenger vi god forskning på det, sier Inger Noer, som er fastlege i tillegg til at hun sitter i programkomiteen i Venstre.
Diagnostikk

Moderator Thomas Axelsen, leder for samfunnspolitisk avdeling i Kreftforeningen, stiller spørsmålet: Har partiene gjort seg noen tanker om diagnostisering av kreftpasienter?
– Tidligere behandlet man brystkreft med en cocktail av cytostatika, og håpet at noe hjalp, med enorme bivirkninger, og der vi er i dag, med diagnostisk og terapautisk skreddersøm, er enorme fremskritt, og det må vi bare heie på, og vi må bare finansiere det, sier Inger Noer, og legger til at selv om det er dyrt, er en økonomisk oppside at vi sparer masse penger på at folk blir friske raskere.
– Det koster penger å begynne å bruke noe, en kostnad som kanskje vil bli lavere etter hvert. Vi må være flinkere til å ta i bruk nye behandlinger raskere, sier Jualianne Ofstad, og legger til at vi bør se til land som er bedre på ta i bruk ny diagnostikk, og til Sverige, der de har satt seg som mål å utrydde livmorhalskreft ti år tidligere enn vi har her i Norge.
– Mer av kreftbehandlingen framover vil ha behov for mer spesialisering, som lymfekreft, og der vil jeg se til EU, der de har et Mission on Cancer som sier at innen 2030 skal 90 prosent av EUs kreftpasienter behandles i et comprehensive cancer center, sier Erlend Svardal Bøe, og legger til at det for Høyre blir viktig å bygge opp disse kreftsentrene i helseregionene i Norge.
– Jeg blir helt svimmel når folk snakker om CRISPR, men vi er langt unna å gjøre det tilgjengelig for folk, og likevel er det the sky is the limit, sier Marthe Scharning Lund, og referer til all ny teknologi som bedrer nettopp diagnostikk.
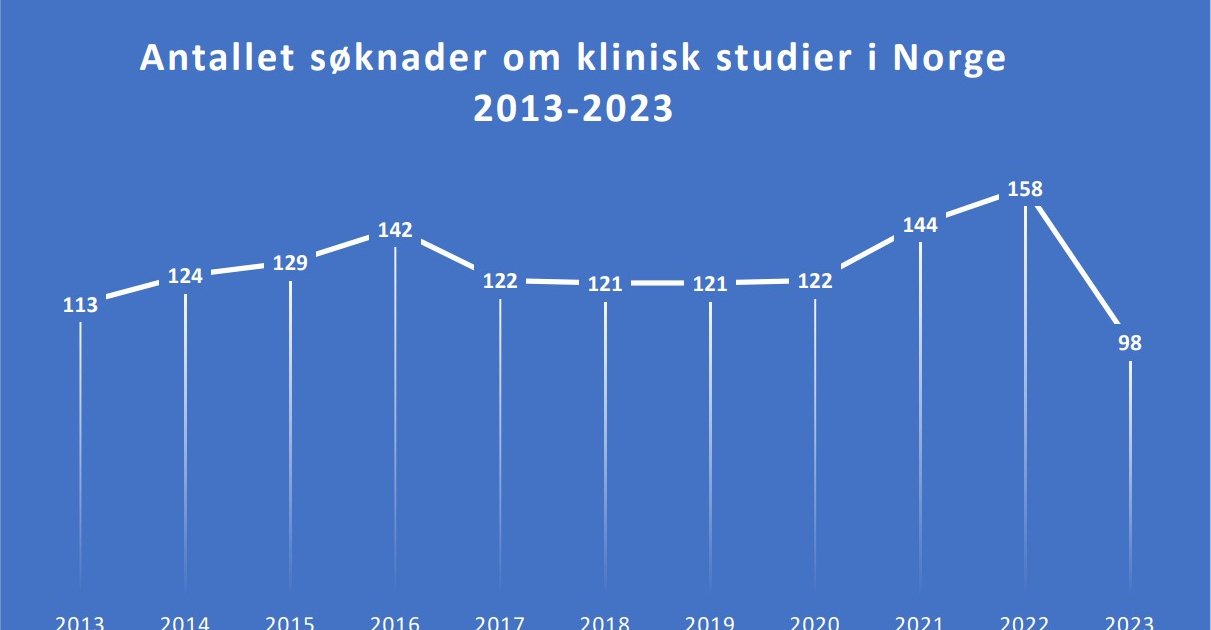
Kliniske studier
I den andre korte videoen snakker MSD Norges Hans Petter Strifeldt om behovet for kliniske studier.
Kan vi gjøre mer for å lykkes med kliniske studier?
Erlend Svardal Bøe var statssekretær i helse- og omsorgsdepartementet da handlingsplanen for kliniske studier ble lagt fram for tre år siden, og han sier at selv om antallet kliniske studier i Norge går ned, bidrar kliniske studier like fullt til at pasientene får raskere tilgang til ny behandling. Han mener at politikerne bør se på en ny handlingsplan, men en annen ting er også viktig:
– Vi bør se på kulturen ute i sykehusene og hvor godt samarbeid vi klarer å ha med industrien og helsenæringen i årene framover.
Inger Noer er enig i at vi bør se på en ny plan for kliniske studier. Hun understreker også at vi må huske på at behandlinger har ulik effekt for menn og kvinner, og at vi derfor også bør se på kvinnehelsemeldingen i denne sammenhengen.
– En ny handlingsplan kunne vært bra for å ha et godt grunnlag, og vi må være konkurransedyktige på dette området. Vi vet at Norge ofte blir nedprioritert av helsenæringen, og det må vi sørge for fra politisk hold, at vi har et godt samarbeid med næringen, sier Julianne Ofstad.
– Vi har et byråkrati i Norge som bruker veldig langt tid på å gi godkjenning for en del utprøvinger, og der har vi en vei å gå, legger Erlend Svardal Bøe til.
Kreftbiobank og helsenæring
I den tredje videoen spiller Ketil Widerberg, daglig leder i Oslo Cancer Cluster, noen næringspolitiske baller over til politikerne, og en om kreftbiobank.
Trenger vi en kreftbiobank?
– Ja, det tror jeg at vi gjør, sier Erlend Svardal Bøe, og legger til at vi må tiltrekke oss kompetansen hjem til Norge, men også skape et bedre hjemmemarked for å utvikle mer helsenæring i landet vårt.
– Hvordan får vi gjort det?, spør Thomas Axelsen.
– Jeg mener det handler om katapult, som vi ikke har hatt på helse, og nå får vi det. Hvordan får vi ideene bedre i system, svarer Bøe.
– Og skal det komme en katapult på helse, kan den godt komme her i Oslo, sier Marthe Scharning Lund, og legger til at det går an å ha fullt fokus på å etablere helsenæring, få ting på marked, og der har vi et godt utgangspunkt i Oslo.
Julianne Ofstad er enig, og understreker at vi da må legge enda mer til rette for bedre offentlig-privat samarbeid.
– Jeg opplever noen ganger at man har en grunnleggende mistillit til hverandre, og det legger ikke et godt grunnalg for samarbeid, sier hun.
– Det at vi prøver å skape innovasjon og næring ut av forskningsresultater er en kjempegod idé! Det er enormt kapitalkrevende, men det må være mulig, sier Inger Noer, og understreker at Venstre er et parti som hele tida har lagt til rette for gündere.
– Og det kan vel få plass i disse partiprogrammene, håper vi, sier moderator Thomas Axelsen.
Samfunnsøkonomien
I den siste videoen presenterer Erland Skogli fra Menon Economics et samfunnsøkonomisk perspektiv på kreft og teknologi. Teknologikomponenten er en mye større del av totalbudsjettet i forsvaret enn i helsesektoren, og skal vi løse helsepersonellkrisen, må vi også ha en økt satsing på teknologi i helse.
Vi går tom for folk før vi går tom for penger, sa Helsepersonellkommisjonen. Har vi tilstrekkelig kriseforståelse for dette i dag?
– Da jeg ble helsebyråd sov jeg nesten ikke om natta da jeg tenkte på hvordan dette skulle gå. Det handler om å bruke penger på dem vi allerede har, gi dem bedre kompetanse og lyst til tå bli i tjenesten, og om bruk av ny teknologi som frigir tid, det handler egentlig om å jobbe på andre måter, sier Marthe Scharning Lund.
– Det er en krise som er her. Vi må se på om tiltak gir en klinisk relevant merverdi for pasienten eller ikke. Vi må slutte med ting som ikke hjelper, avslutter sier Inger Noer.
– Helsevesenet vårt er også en del av totalforsvaret, og funker ikke helsevesenet, kolapser vi fort i en krigssituasjon, for eksempel, sier Julianne Ofstad til slutt.
Velkommen i Arendal
Seminaret Fremtidens kreftpolitikk er del av møteserien Fremtidens kreftbehandling, som i år arrangeres av Kreftforeningen, Janssen, MSD, AstraZeneca og Oslo Cancer Cluster.
Neste frokostseminar i denne møteserien finner sted tirsdag 13. august 2024 under Arendalsuka. Det er også gratis og åpent for alle, og det vil bli strømmet.
Gikk du glipp av frokostseminaret Fremtidens kreftpolitikk 9. april på Litteraturhuset? Du kan se hele seminaret i opptak på Oslo Cancer Clusters YouTube-kanal.
The post Har partiene en kreftpolitikk? first appeared on Oslo Cancer Cluster.


 Oslo Cancer Cluster
Oslo Cancer Cluster 





 Oslo Cancer Cluster
Oslo Cancer Cluster

 ARTBIO / Radforsk
ARTBIO / Radforsk