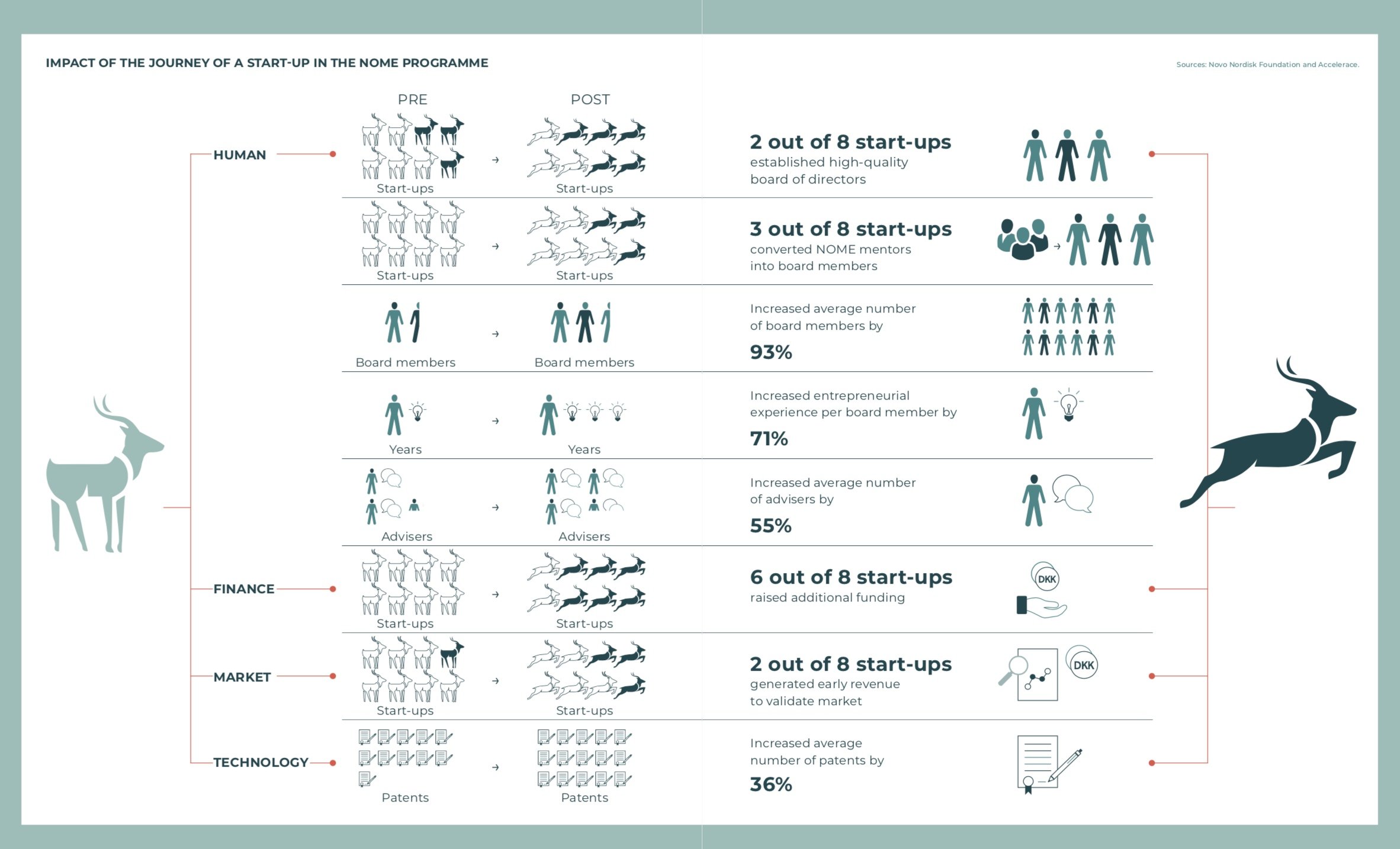
Why a logistics company joined the cluster

Kuehne + Nagel joined Oslo Cancer Cluster last year. Why did a logistics company join a cluster dedicated to cancer treatment?
Kuehne + Nagel is one of the world’s leading logistics providers, and pharmaceuticals are certainly a category of product that requires special care when moved between locations.
This is an interview with Raphael Lømo, the National Manager for Pharma & Healthcare Development Logistics for Kuehne + Nagel in Norway.
“Why did you join a cluster dedicated to cancer treatment?”
“Being one of the leading logistics companies in the pharmaceutical and healthcare industry, we realized that a membership in Oslo Cancer Cluster is beneficial for both the other members and us. The members get access to an international good distribution practice (GDP)-compliant pharmaceutical logistics network and professional support within the pharmaceutical supply chain. At the same time, Kuehne + Nagel gets linked to the currently leading and possible future players in the oncology field, which will help us to increase our understanding and to proactively try to design solutions for the members in this industry. Members can focus on their core competences which is in the R&D field while we offer to take care of the distribution challenges, which is our core competence. Kuehne + Nagel’s membership linked our industries and completed your oncology value chain.”
“We are also very interested in working with start-up companies which are supported by Oslo Cancer Cluster Incubator. It is inspiring to be involved in interesting and innovative projects and at the same time it helps us to keep the finger on the pulse of the pharmaceutical industry. It would not be the first time that we successfully accompanied a start-up by offering pharmaceutical specific supply chain counselling and consulting services.”
“Last but not least, it feels really good to contribute to improve the lives of often very sick cancer patients, which we have been doing for many years in the prostate cancer field. We can identify ourselves with your vision to help patients by accelerating the development of cancer treatments.” Raphael Lømo
“What does logistics innovation have to do with cancer medicine?”
“Well, based on our experience, cancer medicines are often extremely urgent, temperature sensitive and sometimes even classified as dangerous goods shipments, e.g. radioactive. This combination makes it quite challenging to design safe solutions and both visibility, risk control, and reliable handling are the most important factors to protect the integrity of cancer medicines. We constantly work on innovative solutions to improve the level of control of these factors, such as new IT systems and Internet of Things (IoT) real-time tracking devices. ”
“I understand that there are some “pharma shipment enemies” in the logistics industry: Temperature, time, handling and dangerous goods. What is your solution to these challenges in shipping pharmaceuticals?”
“Most importantly, you need a reliable logistics partner which understands the full scope of GDP and the challenges of shipping pharmaceuticals globally. Due to our close relationship to all major airlines, ground handlings agents, and trucking companies, in extreme cases we can customize solutions for very sensitive shipments. Such solutions will be complimented with state of the art tracking technology which transmits both location and other relevant data in real-time to KN Login, our data and IT solution that provides visibility and control of your shipment. There you can follow your shipments 24/7/365. Moreover, a team of trained pharmaceutical logistics specialists can monitor your shipment and provide status updates if required. In case of any deviation of the shipment plan, this global service desk can proactively take action to get your shipment back on track. Our award winning KN PharmaChain solution is the basis for every challenge in the pharmaceutical supply chain industry.”
“We have a vast database that includes the most important information and capabilities of major airlines and ground handling agents at the most important airports around the world. This is a unique database and provides very valuable information in order to plan shipments and conduct Lane Risk Assessments. As an example, with one click we know the capacity for storing pharmaceuticals at certain temperature ranges at warehouses of different airlines and airports all over the world. This tool helps us to analyses shipment processes and mitigate potential risks.”
“Do you have any advice to companies looking to send fragile drugs or other pharmaceuticals?”
“Look for a reliable and experienced logistics partner with a global “owned” network which fully understands the requirements of shipping fragile pharmaceuticals but also follows the Good Distribution Practice (GDP), not only in Norway but globally. We highly recommend to conduct a GDP audit before working with a potential logistics partner. Norwegian logistics companies are not audited by the Norwegian Medicines Authorities and often do not understand and follow the full scope of the GDP guideline. Keep in mind that it is in the responsibility of the pharmaceutical company and not the logistics company that the products are transported under GDP compliant conditions.”
About the company
Kuehne+Nagel is listed on the Swiss stock exchange, but the majority is still owned by Mr. Klaus-Michael Kuehne.
Since 1890, when the business was founded in Bremen, Germany, by August Kuehne and Friedrich Nagel, Kuehne + Nagel has grown into one of the world’s leading logistics providers.
Today, the Kuehne + Nagel Group has some 1,300 offices in over 100 countries, with around 79,000 employees.
The company specialises in seafreight, airfreight, contract logistics and overland businesses, with a clear focus on high value-added segments such as IT-based integrated logistics solutions.
KN PharmaChain is Kuehne+Nagel’s supply chain innovation for pharmaceutical and healthcare shipments.