Promising early data from Exact Therapeutics’ study

The company is investigating a new ultrasound technology for improved drug delivery.
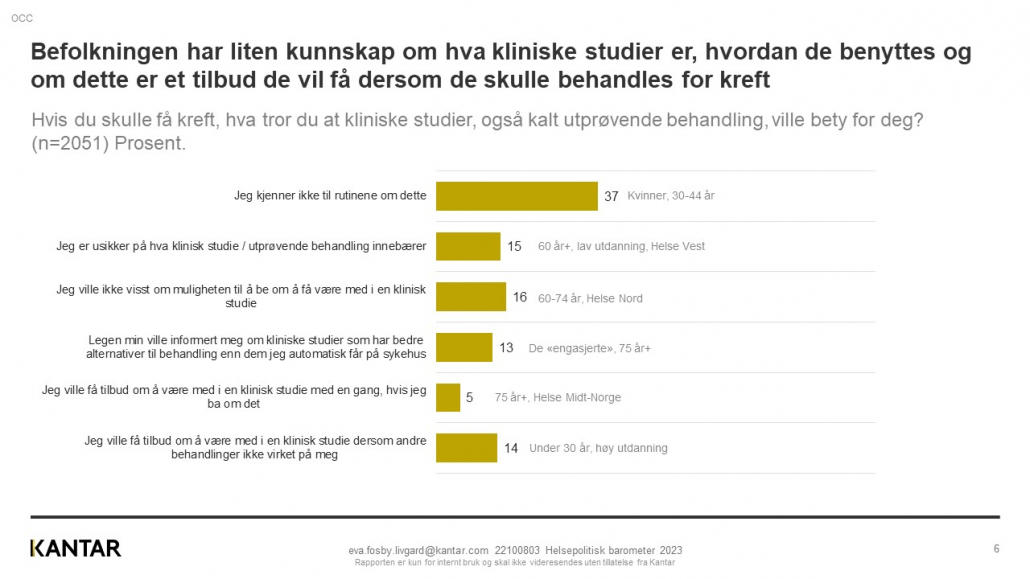
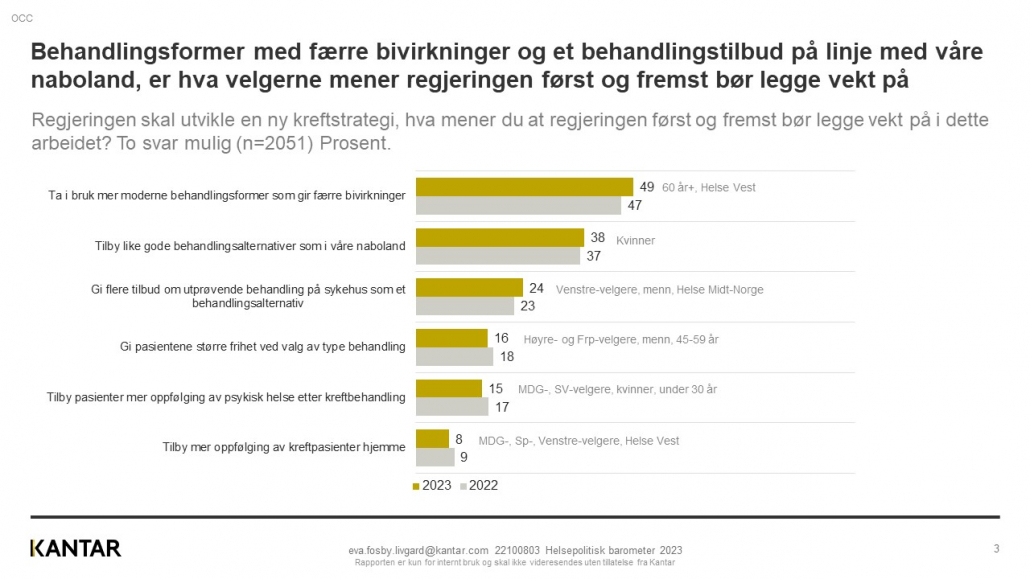
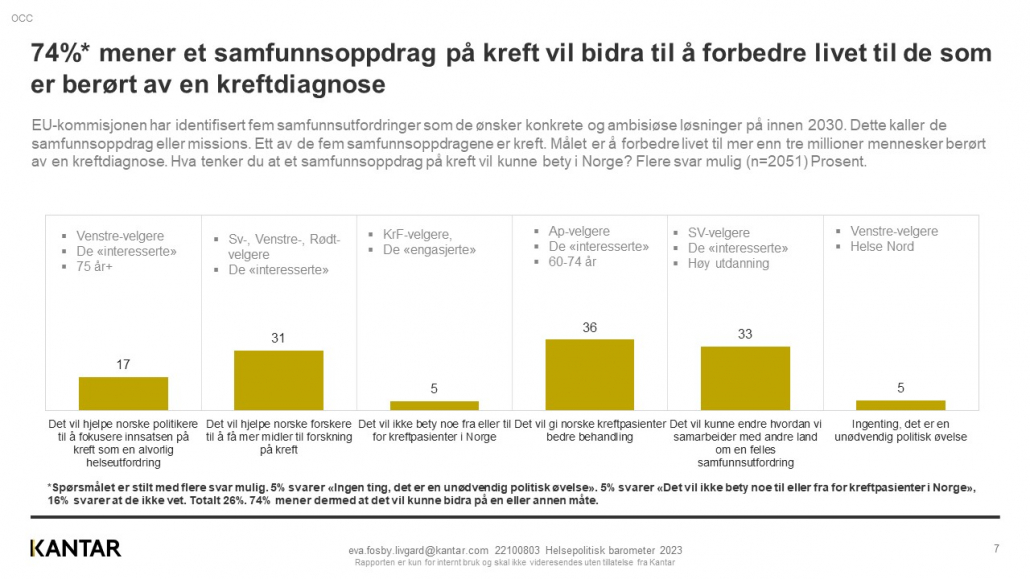
Exact Therapeutics shared early data from two patients treated in the clinical study ACTIVATE at a Science and Technology event in Oslo this week.
The patients have colorectal cancer with liver metastases, a patient group with a high unmet medical need. They receive chemotherapy in combination with the company’s technology called ACT (short for Acoustic Cluster Therapy). The early results show that the patients had better responses to chemotherapy when their tumours were exposed to ACT.
“Results of the Phase I study have given us several insights, most importantly that the treatment did not reveal any unexpected side effects either on its own or worsen the side effects of chemotherapy in the treated patients. The results also suggest a positive tumour response associated with the ACT treatment. This trial will set the stage for diversifying the use of this technology platform to a wide range of systemic therapies across multiple tumour types,” commented Prof. Dr. Udai Banerji, the principal investigator of the ACTIVATE study.
Seven patients have been recruited to the study, currently active at The Royal Marsden Hospital and Newcastle Hospital. The company is also collaborating with radiologists at Oslo University Hospital to open another study site for Norwegian patients.
What is ACT?
Acoustic cluster therapy is a method for improved drug delivery, by using ultrasound technology and the company’s proprietary product consisting of microbubbles and microdroplets. The microbubbles and microdroplets have opposite charges and therefore form small clusters, which are injected into the patient’s blood.
When these clusters are exposed to ultrasound vibrations, the droplets evaporate into the bubbles and turn into larger “ACT bubbles”. These are temporarily trapped in the patients’ capillaries, the smallest blood vessels in the body. The ACT bubbles can then be manipulated with a different ultrasound wavelength that makes them oscillate. This stretches the wall of the blood vessel and creates shear forces and streaming in the tissue, so that the cancer drug can be delivered effectively to the tumour.
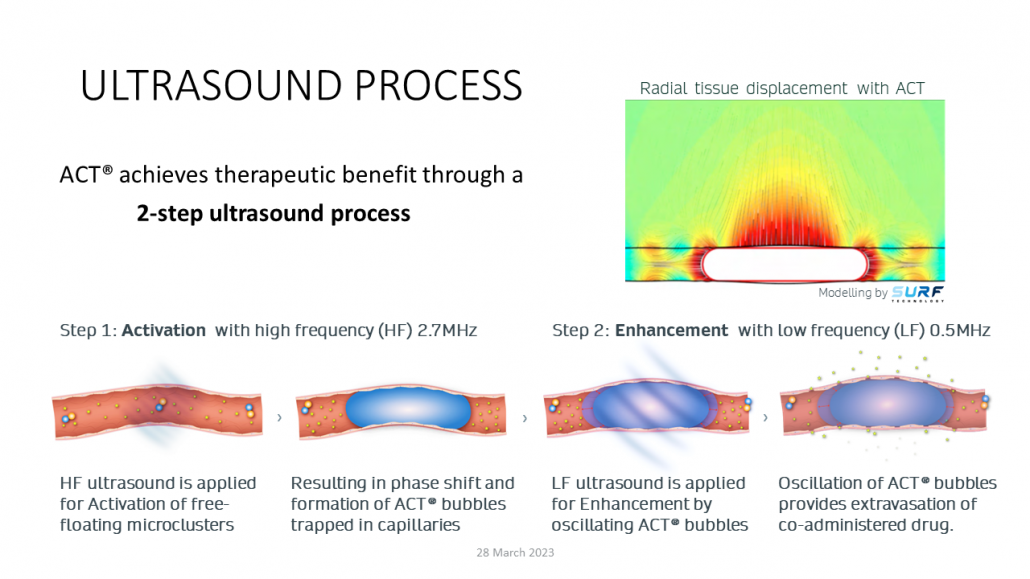
“Targeted ultrasound is an emerging treatment modality with a lot of interest. You need better methods for efficient delivery of drugs to the disease areas. Targeted ultrasound with ACT is non-invasive and can potentially be done for a number of treatments and indications,” commented Per Walday, CEO of Exact Therapeutics.
The Norwegian ultrasound story
There is a solid Norwegian research tradition within ultrasound, both in medical technology and pharmaceutical diagnostics.
For example, the company Vingmed Ultrasound was built on Norwegian science and technology, and established as a start-up in 1986 developing medical ultrasound diagnostic equipment. The company was later acquired by GE Healthcare and this segment today generates 3 billion dollars in revenue.
Another success story is the company Nycomed, which was also founded on deep science in Norway, and created contrast agents in a variety of forms for diagnostic purposes. Nycomed ended up achieving market leadership and was acquired by GE Healthcare. Today, this is a 2-billion-dollar revenue business.
“Norway is a true global leader in this field, not only in technology and science, but also in business. The medtech and pharmaceutical components are key to us. Exact Therapeutics was spun-off from the pharma business in GE Healthcare. We have agreements with GE Healthcare on development and manufacturing. These are the two building blocks and the foundation for Exact,” commented Anders Wold, Chairman of the Board, Exact Therapeutics.
Potential with immunotherapy
The potential of the technology to enhance the effect of a range of cancer immunotherapies will now also be explored, thanks to a grant of NOK 16 million from the Norwegian Research Council.
“The tumour microenvironment plays a key role in the response to cancer therapies, especially immunotherapy. The majority of cancer patients still do not respond to immunotherapy. The holy grail in this field is to make cold tumours hot and the key is to modify the tumour microenvironment. We hope to achieve this with the ACT treatment,” commented Walday.

Per Walday, CEO, at Exact Therapeutics’ Science and Technology event. Photo: Sofia Linden / Oslo Cancer Cluster
The research project will be performed in collaboration with the Norwegian University of Science and Technology (NTNU), the Institute of Cancer Research at the Royal Marsden Hospital in London and the Translational Genomics Research Institute, part of City of Hope, in Phoenix.
The post Promising early data from Exact Therapeutics’ study first appeared on Oslo Cancer Cluster.



 Pixabay
Pixabay

